Main highlights:
- Pfizer and Sangamo Therapeutics come together for Gene therapy
- Pfizer and Sangamo’s statements
 Source: Google Images
Source: Google Images
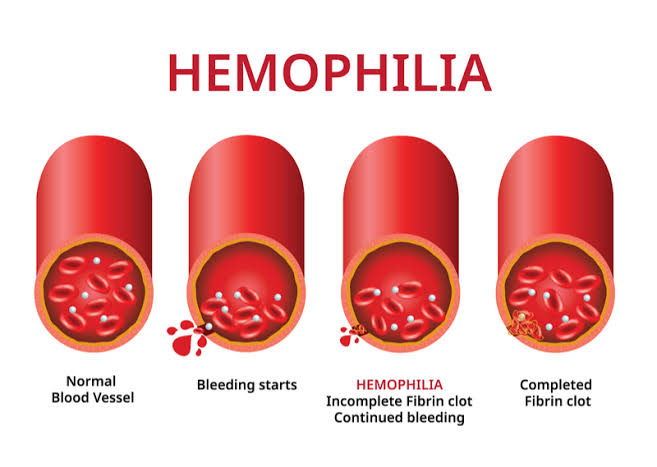
A Phase 3 trial of a gene treatment developed by Pfizer and Sangamo Therapeutics for the blood condition haemophilia A will shortly resume, nearly a year after it was put on hold due to safety concerns.
The businesses specifically stated Thursday night that they intended to begin patient enrollment in the study this month and resume treatment administration in October.
The trial was initially put on hold last fall because of worries about blood clotting and safety. Giroctocogene fitelparvovec, a type of gene therapy, is used to treat persons with severe haemophilia A who are either deficient in or have low amounts of a crucial blood clotting protein.
Some patients experienced higher-than-normal levels of that blood clotting protein as a result of treatment, and many were consequently prescribed oral blood thinners. Later, Pfizer revealed that one patient had higher clotting protein levels combined with deep vein thrombosis in the leg.
After Pfizer and Sangamo changed their trial protocol, the Food and Drug Administration lifted a clinical hold it had placed in May, enabling testing to continue.
The two firms now anticipate a slightly later resumption after having hoped to resume dosing around the end of September. Trial data, which had been anticipated for 2023, is now anticipated in early 2024, Pfizer announced on Thursday.
Some patients experienced higher-than-normal levels of that blood clotting protein as a result of treatment, and many were consequently prescribed oral blood thinners. Later, Pfizer revealed that one patient had higher clotting protein levels combined with deep vein thrombosis in the leg.
After Pfizer and Sangamo changed their trial protocol, the Food and Drug Administration lifted a clinical hold it had placed in May, enabling testing to continue.
The two firms now anticipate a slightly later resumption after having hoped to resume dosing around the end of September. Trial data, which had been anticipated for 2023, is now anticipated in early 2024, Pfizer announced on Thursday.
Here’s what companies have to say:
Disclosure Notice from Pfizer:
This release’s information is current as of September 22, 2022. Pfizer disclaims any need to revise any forward-looking statements in this statement in light of new information, unanticipated developments, or other factors.
The phase 3 AFFINE study, including the anticipated timing of active trial sites, and an investigational haemophilia A therapy called giroctocogene fitelparvovec are two examples of forward-looking information in this release. These statements involve significant risks and uncertainties, which could cause actual results to materially differ from those expressed or implied by such statements.
the ability to meet anticipated clinical endpoints, commencement and/or completion dates for our clinical trials, regulatory submission dates, regulatory approval dates, and/or launch dates, as well as the possibility of unfavourable new clinical data and further analyses of existing clinical data; risks associated with interim data; the risk that clinical trials will not be successful. If and when drug applications for giroctocogene fitelparvovec may be submitted in any jurisdictions for any conceivable indications;
if and when drug applications for giroctocogene fitelparvovec may be submitted in any jurisdictions for any conceivable indications; whether and when regulatory authorities in any jurisdictions may approve any such applications will depend on a variety of factors, such as deciding whether the product’s benefits outweigh its known risks, determining the product’s efficacy, and determining whether giroctocogene fitelparvovec will be commercially successful if approved
Giroctocogene fitelparvovec may be available or have commercial potential depending on regulatory authority decisions regarding labelling, manufacturing processes, safety, and/or other issues. There are also concerns about COVID-19‘s potential effects on Pfizer’s operations, financial results, and business.
 Source: Google Images
Source: Google Images
Pfizer’s Annual Report on Form 10-K for the fiscal year ended December 31, 2021, its subsequent reports on Form 10-Q, including those therein titled “Risk Factors” and “Forward-Looking Information and Factors That May Affect Future Results,” as well as its subsequent reports on Form 8-K, all of which are filed with the U.S. Securities and Exchange Commission, provide a more detailed explanation of risks and uncertainties.
Disclosure Statement for Sangamo Therapeutics:
Sangamo’s current expectations are discussed in forward-looking statements in this release. The plans and timing for the Phase 3 AFFINE clinical trial’s active trial sites, including the restart of patient enrollment, expectations for the anticipated timing of dose resumption and data readouts, and other statements that are not historical facts are all examples of forward-looking statements.
These statements contain risks and uncertainties that are hard to foresee and are not promises of future performance. Actual results for Sangamo could significantly and negatively differ from those projected in these forward-looking statements. Risks and uncertainties associated with the COVID-19 pandemic and its impact on the global business environment, healthcare systems, and the operations of Sangamo and Pfizer, including patient enrollment and clinical trial operations, are just a few of the factors that could result in different results in reality.
the unpredictable timing and results of clinical trials, including the possibility of patient durability of treatment effects in the Phase 3 AFFINE experiment; production of products and product prospects; marketing of authorised products; the unpredictable regulatory approval procedure for product candidates across different regulatory agencies; the possibility of technical advancements eliminating methods used by Pfizer and Sangamo in giroctocogene fitelparvovec; possible termination of Pfizer’s collaborative agreement with Sangamo, suspension of the giroctocogene fitelparvovec development, or both.
Sangamo’s inability to fully develop, obtain regulatory approval for, and commercialise its product candidate, giroctocogene fitelparvovec, as well as other risks and uncertainties are detailed in Sangamo’s filings with the U.S. Securities and Exchange Commission, including its Annual Report on Form 10-K for the year ended December 31, 2021, as supplemented by Sangamo’s Quartial Report on Form 10-Q for the same period. This release’s contents are current as of September 22, 2022, and Sangamo makes no commitment to update any forward-looking statements unless required by applicable legislation.





