@media (max-width: 1200px) {
}.ns-inline:not(.ns-columns) .ns-buttons-wrapper {
justify-content: center;
}body .ns-inline:not(.ns-columns) a.ns-button, body .ns-inline .ns-total-share-count {
margin: 0px 5px 10px 5px;
}47FacebookMessenger593PinterestTwitterWhatsAppEmail640SHARESbody .ns-pinterest-image{display:block;position:relative;margin:0;padding:0;line-height:0}figure>.ns-pinterest-image{height:100%;width:100%}body .wp-block-image .ns-pinterest-image+figcaption{display:block}body .ns-pinterest-image-button{opacity:0;transition:.3s;position:absolute;height:18px;max-height:18px;width:auto!important;padding:10px;cursor:pointer;background:#c92228;color:#fff;font-size:16px;line-height:18px;z-index:1;text-decoration:none;box-sizing:content-box;top:10px;left:10px}body .ns-pinterest-image-button:hover{background:#b51f24}body .ns-pinterest-image-button:visited{color:#fff}body .ns-pinterest-image:hover .ns-pinterest-image-button{opacity:1}body .ns-pinterest-image-button svg{width:18px;height:18px;vertical-align:middle;pointer-events:none}
 Pin
Pin
Much of America is experiencing a cold snap, the coldest weather in decades (according to the evening news).
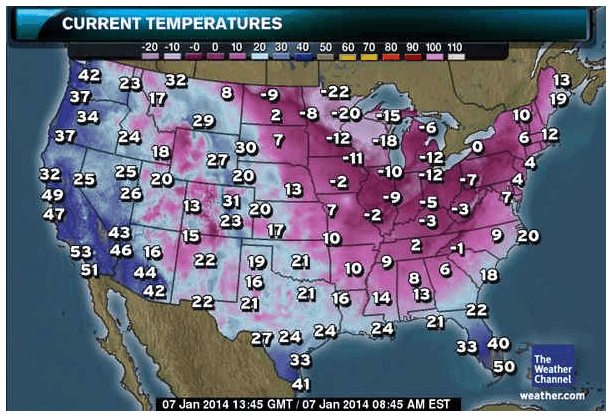 Pin
Pin
I personally don\’t remember ever seeing sub-zero temperatures, so this is sort of a big deal.
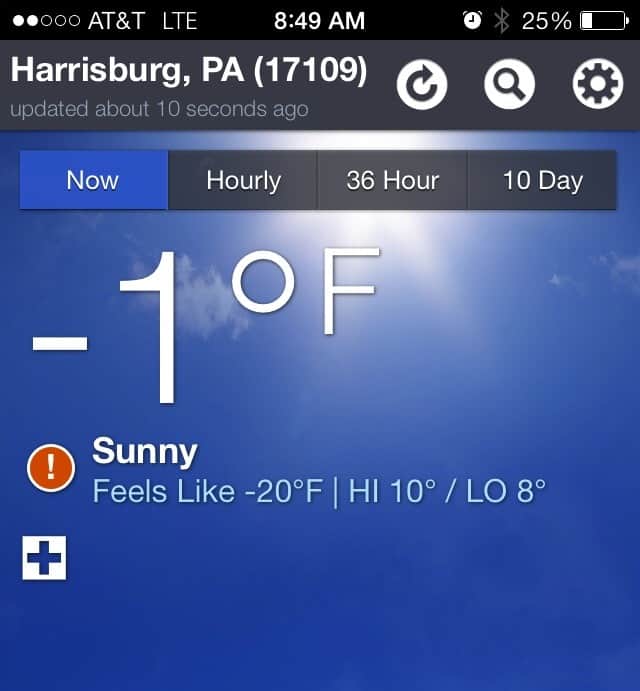 Pin
Pin
My children, who love to go outside every day – and especially go! play! in! the! snow! – are stuck inside, for skin will begin to freeze in minutes at these temperatures.
Schools are all closed; art class is cancelled. Even Joe\’s office is closed today, on account of the bitterly cold weather. Everyone is staying indoors and keeping warm.
{Say a prayer for those people without reliable heat, for I know not what they are doing right now.}
We did venture outside for two minutes for one little science experiment. (And that was long enough because it is colder outside than I even imagined. Cold enough to make your bare skin ache almost instantly.)
Yesterday, I saw Linda at The Joyful Journey do this science experiment on her blog. I wanted to know How?! and Why?! and How cold does it have to be?! but she wasn\’t sure. So I decided to do some research and share the whole thing with you, in case you want to wow your own children.
Instant Snow – A Science Activity for a Cold, Cold Day
At Linda\’s suggestion, I googled how to make snow from boiling water, and I found lots of information. Here are the basics:
- The day must be very cold and dry. (We tried it at -1, as you can see above, but would have tried at 5 or 6, too. I don\’t know how warm it can be and still work.)
- Boil water. (Boiling water should be done only by an adult.)
- Take the boiling water outside. (Boiling water should be handled and transported only by an adult.)
- Throw the boiling water up and away from you.
- Watch the snow.
Here\’s our first attempt.
As you can see, Joe threw the water too hard, and the pan was too small.
For attempt #2, Joe used a much larger pot with twice as much water, and he didn\’t throw it up as hard.
He also tried to run away from what he thought was raining boiling water and incurred a sizable wound on his knee. The water, as it fell, was cold.
Because it\’s that cold outside.
The Science Behind Turning Boiling Water into Snow
Hot Water
You may be thinking it would be easier to freeze cold water, but that\’s not the case. You want the water to be hot, boiling even.
The molecules in hot water are moving fast – the hotter it is, the faster they\’re moving. Quickly moving molecules jump around and become water vapor, so when you throw them up into the air, you\’re really throwing up a hot water and a lot of hot gas (the water vapor, which is mixed in with the water as bubbles and also forming as the hot water evaporates).
The gas freezes instantly in a process called deposition, and it breaks the hot water into tiny liquid bubbles, which also freeze quickly into tiny ice crystals. The tiny ice crystals form a cloud just like the thin, wispy cirrus clouds you see up high on sunny days.
Incidentally, if you had a water gun that would safely spray boiling water, it would freeze impressively.
The closer your water is to a full, rolling boil, the more of it will freeze. In the time it took for our water to travel from the stove, down the stairs, through the house, and outside, it cooled a bit, leaving about half of the water to fall as rain onto the driveway. (Hence Joe ran backwards away from it and skinned his knee.)
Cold water won\’t work in this experiment because it produces practically no water vapor. The molecules are moving slowly, very little water is evaporating, and it will take minutes or longer to freeze. It would not have time to freeze in the air.
Relative Humidity
This experiment works best when the humidity outside is very low. When the humidity is low, there is very little water vapor in the air. This allows more water vapor to escape from your boiling water into the air. The more water vapor that can escape, the more ice crystals will form.
Fortunately, most icy cold days are dry, with low humidity levels.
Outside Temperature
Obviously, the warmer it is, the less of your liquid water will freeze. At 10 degrees Fahrenheit, almost none of your water will freeze (and you will end up with rain). At 0 degrees Fahrenheit, most of our water froze. I have seen videos of other people throwing boiling water into the air at colder temperatures with even better results. We may try it again this afternoon, when the temperature is supposed to closer to 5, just to see what happens.
The reason it won\’t work at slightly warmer (though still freezing) temperatures has to do with the principle of heat transfer. When two substances are mixed, their temperatures try to come to equilibrium – a stable state where both substances are the same temperature.
In this case, you\’re mixing water (above 200 degrees F) with air (below 0 degrees F). Because there\’s a big difference in their temperature, the water cools very quickly. Because of the above water vapor and tiny particles principles (and the fact that there\’s a whole lot of air and very little water), the water cools down much more quickly than the air heats up.
Impure Water
Ice crystals form most easily when the water contains impurities. Atoms or molecules not normally found in water (chlorine, fluorine, salts, etc) act as seeds for ice crystals to form. The more impurities in the water, the quicker ice crystals begin to form in the liquid water. Tap water or rain water from a bucket would work better in this case than filtered, purified, or distilled water. The latter will not make ice crystals nearly as readily as the former.
This process is similar to how ski resorts make their snow.
They simply spray a very fine mist of hot water into cold air. It turns into ice crystals and falls to the ground as something that looks like snow.
What\’s the temperature at your house today?
@media (max-width: 1200px) {
}.ns-inline:not(.ns-columns) .ns-buttons-wrapper {
justify-content: center;
}body .ns-inline:not(.ns-columns) a.ns-button, body .ns-inline .ns-total-share-count {
margin: 0px 5px 10px 5px;
}47FacebookMessenger593PinterestTwitterWhatsAppEmail640SHARES





